Calcium (Ca)
Calcium receives a lot of flack in the health industry. I hope that this post brings some clarity to its uses in nutrition and the benefits of calcium supplementation.
Calcium is the fifth most abundant mineral on Earth and is the top most abundant element in the human body. It is essential in the biosphere for all living beings on Earth (source). Its name comes from the Latin word "calx" (genitive calcis) or "limestone" (source) and has been used as a structural building material as far back as 14,000 BC, and is used as "plaster of Paris" (calcium sulphate) for the making of casts for broken bones and even in art (source).
Calcium was first discovered in England, 1808. Humphry Davy was attempting to reduce moistened lime, the mineral - not the fruit, by electrolysis just as he had done with sodium and potassium - however, he was not successful. Consequently, he tried a mixture of lime and mercury oxide. Even though this produced an amalgam of calcium and mercury, his attempt and result was not enough to confirm that he had indeed discovered a new element. A similar experiment had been conducted by Jöns Jacob Berzelius who had also obtained the amalgam. In another attempt, Davy tried using even more lime in the mixture and produced more of the amalgam which he was then able to distil off the mercury leaving the element Calcium (source).
Calcium is one of the body's main electrolyte minerals. Over ninety per cent of the calcium in the body is stored in the bones and teeth, which function as reservoirs which calcium can be withdrawn as required for extra-skeletal functions. When combined with phosphate, a substance called hydroxyapatite is formed. Hydroxyapatite is the mineral portion of bones and teeth, in humans and animals, and some coral.
Calcium has many functions in the body. Some of them include inhibiting thyroid-releasing hormone (TRH) (source) and increasing insulin secretion (source, source). It inhibits the sympathetic nervous system (source) and is required for phosphorus metabolism as well as energy production in the Krebs cycle (source, source).
Calcium helps regulate cell permeability, acid-base balance (source), hormone secretion (source, source), cell division and differentiation (source, source), stem cells (source), and osmotic balance (source). It helps muscles relax and slows nerve transmission while calming the heart rate, as well as aiding in the prevention of fluid loss from both cells and the blood.
Calcium provides structural strength to bones and contributes to critical functions which are necessary for the physiology and biochemistry of living organisms as a whole and the cell. It is essential for signal transduction pathways (source, source) where it functions as a second messenger (source), the release of neurotransmitters from neurons (source, source), in contraction of all muscle cells (source), and in fertilization (source). Many enzymes require calcium ions as a co-factor such as those of the blood-clotting cascade (source).
The most important activities of cells are regulated by calcium. It is found in virtually every cell throughout the body and is considered to be a biological messenger that is responsible for carrying signals to target activities and cells through specific calcium channels. Whether it be from the expression of genes, for heart and muscle contraction and other motility processes, or for diverse metabolic pathways involved in the generation of cell fuels, calcium is essential. When blood levels of calcium decline, calcium is drawn from the bones, which function as a reservoir of the element. Thus a lifelong dietary deficiency of calcium manifests in later life as osteoporosis, a common health problem in nearly every individual, that is mistakenly assumed to occur with age.
Calcium can be found both inside and outside of the cell. Extracellular calcium is important for maintaining the potential difference across excitable cell surface (so-called membranes), as well as for proper bone formation. While intracellular calcium is stored in organelles which repetitively release and then re-accumulate Ca2+ ions in response to specific cellular events. Intracellular storage sites include the mitochondria and the endoplasmic reticulum.
Mechanism of action:
Calcium is a divalent metal essential for maintenance of the neuromusculoskeletal system. It influences cell membrane and capillary permeability and is important for bone strength, blood coagulation, and electrical conduction in nerves and the heart. Hormones, or first messengers, interact with cell membrane receptors and produce signals that generate second messengers inside cells. Calcium and the calcium-calmodulin complex, like cyclic adenosine monophosphate (cAMP), can act as second messengers. When calcium combines with calmodulin, this small regulatory protein undergoes a conformational change that allows it to interact with several enzymes, resulting in an increase in the catalytic activity of various protein kinases, including those that regulate membrane permeability to calcium and control the intracellular contractile proteins - actin and myosin. About 99% of calcium is stored in bones as hydroxyapatite.
In the electrical conduction system of the heart, calcium replaces sodium as the mineral that depolarizes the cell, proliferating action potentials. In cardiac muscle tissues, sodium influx commences an action potential, but during potassium efflux, the cardiac myocyte experiences calcium influx, prolonging the action potential and creating a plateau phase of dynamic equilibrium.
The FDA, Calcium Intake and Disease.
Calcium intake is a major determinant of peak bone mass, and the risk of osteoporotic fractures is strongly influenced by bone mass. Low calcium intake has also been implicated in the development of various chronic conditions including: hypertension, colon cancer, nephrolithiasis, and even premenstrual syndrome (source); calcium supplementation has been used in the prevention of these diseases. Calcium is an essential component of antiresorptive agent therapy for osteoporosis (source)
NOTE: (Since the elemental constituents of bone are not purely calcium, it has been my experience that the other elements that are necessary for bone health should also be supplied in the case of rebuilding bone).
As a general rule, dietary supplement labeling and marketing cannot legally state disease prevention, or treatment claims. However, for some foods and dietary supplements, if the FDA has reviewed the science and concludes that there is significant scientific agreement, it allows for the publication and advertising of specifically worded health claims.
In 2005 the FDA approved a Qualified Health Claim (QHC) for calcium and hypertension, with the suggested wording "Some scientific evidence suggests that calcium supplements may reduce the risk of hypertension. However, FDA has determined that the evidence is inconsistent and not conclusive."Although evidence for pregnancy-induced hypertension and preeclampsia was considered inconclusive (source), the FDA has also established a QHC for calcium and colon cancer, with the suggested wording "Some evidence suggests that calcium supplements may reduce the risk of colon/rectal cancer, however, FDA has determined that this evidence is limited and not conclusive." Evidence for breast cancer and prostate cancer was considered inconclusive (source), and QHC regarding calcium supplementation protecting against kidney stones or against menstrual disorders or pain were denied (source, source).
An initial ruling allowing a health claim for calcium dietary supplements and osteoporosis was later amended to include calcium and vitamin D supplements, effective January 1, 2010. In order to qualify for the calcium health claim, a dietary supplement must contain at least 20% of the Reference Dietary Intake; which for calcium means at least 260 mg of elemental calcium per serving (source).
Examples of allowed wording are shown below:
"Adequate calcium throughout life, as part of a well-balanced diet, may reduce the risk of osteoporosis."
"Adequate calcium as part of a healthful diet, along with physical activity, may reduce the risk of osteoporosis in later life."
"Adequate calcium and vitamin D throughout life, as part of a well-balanced diet, may reduce the risk of osteoporosis."
"Adequate calcium and vitamin D as part of a healthful diet, along with physical activity, may reduce the risk of osteoporosis in later life."
Signs and Symptoms of Calcium Deficiency:
- Arthritis
- Bone spurs
- Brittle fingernails
- Cognitive impairment
- Delusions
- Depression
- Eczema
- Hyperactivity
- Hypertension
- Insomnia
- Irritability
- Limb numbness
- Muscle cramps
- Nervousness
- Neuromuscular excitability
- Osteomalacia
- Osteopenia/Osteoporosis
- Palpitations
- Paraesthesia
- Periodontal disease
- Pica
- Rickets
- Retarded growth
- Tetany
- Tinnitus
- Tooth decay
Signs and Symptoms of Calcium Excess:
- Aches and pains
- Arthritis
- Anorexia
- Aphasia
- Ataxia
- Depression
- Irritability
- Insomnia
- Memory loss
- Muscle weakness
- Muscle tightness and cramps
- Psychosis
- Arrhythmia
- Fat intolerance
- Fatigue
- Flaccid tissues
- Gastric ulcers
- Growth retardation
- Hypertension
- Kidney disease
- Liver impairment
- Weakness
Food sources:
Good sources of calcium are milk, cheese, dolomite, bone meal, sesame seeds, dark green vegetables, dried legumes, sardines, tuna, and salmon. The concentration of calcium in foods varies. One cup of reconstituted nonfat dry milk contains 375 mg of calcium; a cup of low-fat, skim or whole milk has 290 to 300 mg; a cup of yogurt has 275 to 400 mg; a cup of low-fat cottage cheese has 154 mg; and a cup of part-skim ricotta cheese has 680 mg. One ounce of Swiss cheese contains 272 mg, and one ounce of cheddar has 204 mg.
Among non-dairy sources, 3 ounces of sardines with bones contains 370 mg, and an equivalent amount of canned salmon with bones contains 285 mg calcium. The best vegetable source for calcium is tofu, where 4 ounces contains 154 mg.
Botanically, cereal grains are the fruit of the plant, which accumulates the least amount of calcium (source). Absorption of calcium from dairy products is the most efficient way to get the RDA in modern diets. However, it is unclear whether dairy foods promote bone health in all populations and whether all dairy foods are equally beneficial (source). A review of 57 outcomes of the effects of dairy foods on bone health indicated that 53% were not significant, 42% were favourable, and 5% were unfavourable. In 21 stronger-evidence studies, 57% of outcomes were not significant, 29% were favourable, and 14% were unfavourable. The authors concluded that foods such as milk and yogurt are likely to be beneficial; although other foods, such as cottage cheese, may adversely affect bone health. A systematic review of 13 articles published between 1926 to 2018 suggests that supplementing the usual diet with dairy products significantly increases bone mineral content during childhood (source). Many studies suggest that milk is essential for healthy bones (source, source, source) in all stages of development, children (source, source, source), adults (source, source. source) and elderly (source, source, source).
The amount of dietary fat consumed relative to dietary fibre, appears to have an important role in determining differences in calcium absorption (source). Fractional calcium absorption is positively associated with dietary fat intake, body mass index, serum 1,25 dihydroxyvitamin D, and parathyroid hormone concentrations. It is inversely associated with total calcium intake, dietary fibre intake, alcohol consumption, and, to a lesser extent, with physical activity and symptoms of constipation. Dietary fat, fibre, and alcohol are independent predictors of calcium absorption. Calcium absorption varies from 17% to 58% among individuals (source). Diets that have an excess of sodium, protein, or both, increase the dietary requirement of calcium. Sodium intake is a significant determinant of urinary calcium, as sodium intake increases, urinary calcium levels increase (source). Protein produced a similar effect, in that as protein intake doubles, urinary calcium increases up to 50% (source, source, source, source, source). However, this increased need for calcium with dietary protein does not seem to be due to the phosphorus content of meat (source). There are still no definitive nutrition intervention studies, that I am aware of, that show a detrimental effect of a high protein diet on the skeleton.
Lacto-vegetarians are able to meet recommended calcium intakes and do not have compromised bone mineral density. Few other foods provide concentrated sources of absorbable calcium (source). Although it is possible to obtain calcium balance from a plant-based diet in a Western lifestyle, the quantity of vegetables required to reach sufficient calcium intake, makes an exclusively plant-based diet (vegan) impractical for most individuals - unless fortified foods or supplements are included (source). As an example of the difficulty of reaching the RDA with plants, while the calcium found in broccoli is much more bioavailable than that of milk, it would require 4.5 cups of broccoli to equate to a single glass of milk (source). Thus, dairy sources of calcium, such as milk, are much more feasible and practical.
For people with lactose intolerance, which is about 65% of the global population (source), it would be wise to supplement the diet with calcium supplements, or perhaps consume lactose-free dairy products (source).
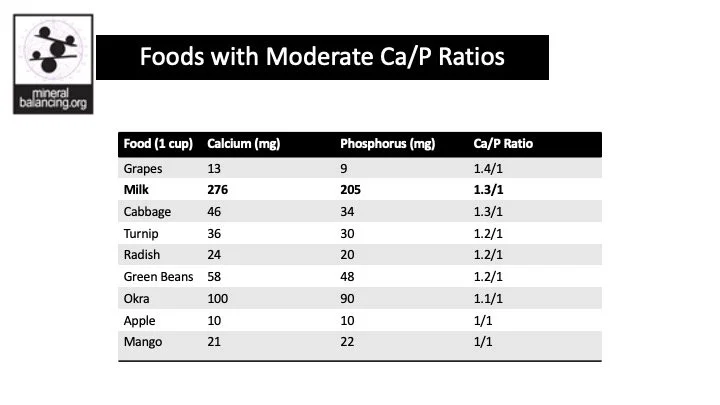
Ideal Dietary Ratio of Ca/P is 2:1
Click on the images to see the Calcium to Phosphorus ratios in foods.
Dose:
The recommended daily allowance (RDA) for calcium is between 800 and 1400 mg as of 13/07/2019.
The recommended intake increases on a sliding scale:
200mg/day for babies under 6 months of age (source).
250mg/day for babies between 7-11 months of age (source.
5-700mg/day for infants and children up to the age of 3 years (source)
At least 800mg/day for children aged 4 to 8 years (source)
800 to 1300mg/day for children older than 9 years and adults (source)
An intake of 1300mg per day of calcium has been proposed for pregnant and lactating women (source).
The supplementary range for calcium is 1000 to 2000 mg. The RDA for calcium has been revised upward, and it appears that this trend may continue in the future. The diet of Paleolithic (stone age) human adults has been estimated to have contained from 50 to 75 mmol of calcium (2000 to 3000 mg)/d, at least twice the median calcium intake of present-day US adults (source).
One factor determining the RDA of calcium is urinary excretion. Calcium excretion, below the dietary threshold for calcium, correlates negatively with peak bone mass. The dietary threshold for calcium in teenagers is about 1500 mg daily. Overall, urinary calcium levels reach a plateau when calcium intake is between 500 and 2000 mg. Urinary losses of calcium can be minimized by the administration of calcium supplements, in doses in excess of 500 mg, and by avoiding foods and beverages with high sodium, protein, sugar, and/or caffeine content. An additional 6 mg of calcium is lost when caffeine intake reaches 175 mg/day, and 1 mg is lost for every gram of protein consumed (source, source). Some studies have shown that two-three+ cups of coffee may result in bone loss in individuals with low calcium intake (source, source).
Another factor influencing the RDA for calcium is calcium absorption. Calcium supplements should not be taken with fibre-rich meals. Phytates and oxalates found in bran, whole-grain cereals, and bread, impede absorption. On the other hand, lactose enhances absorption, and dairy products are particularly good sources. Absorption of calcium is increased when supplements are taken in divided doses. Calcium supplements are best taken 60 to
90 minutes after meals, unless the person has achlorhydria, in which case supplements are best taken with meals. Calcium is better absorbed from lactate, citrate, or gluconate rather than from carbonate; this is particularly important in elderly people and those with hypochlorhydria. Although inexpensive, calcium carbonate is of limited value as either a calcium supplement or an antacid. Any benefit derived from calcium carbonate reducing gastric irritation by neutralizing gastric acid, is compromised by calcium’s stimulation of gastrin production.
For effective and accurate dosing, note that not all calcium supplements are equal. 1,000mg of calcium lactate or calcium carbonate differ in the total amount of elemental calcium. The RDA is based off the total elemental content and not the amount of chelate. For example (source):
Calcium carbonate, contains 40 percent elemental calcium. It’s best absorbed when taken with food because it needs stomach acid to be absorbed.
Calcium lactate contains 13 percent elemental calcium.
Calcium gluconate contains 9 percent elemental calcium
Calcium citrate contains 21 percent elemental calcium. Calcium citrate can be taken with or without food. It is useful for people with inflammatory bowel disease (IBD), achlorhydria, and some absorption disorders.
Clinical Uses:
Calcium intake levels greater than 2500 mg/day are not recommended (source). For osteoporosis prevention, 1200 mg of calcium per day is minimally required. A daily intake of 400 to 600 IU of vitamin D is also recommended which can be achieved through sun exposure, diet, and/or supplementation to ensure adequate calcium absorption (source). Higher intakes of calcium are associated with increased bone density and a reduced risk of gastrointestinal tract cancers. The risk of being obese increases 6-fold as one proceeds from the highest to the lowest quartile of calcium intake according to the National Health and Nutrition Examination Survey (NHANES) database (source).
Calcium absorption and bone deposition vary at different stages of the life cycle, peaking in early puberty and decreasing gradually after menarche (source). A study of 22 pairs of twins, showed that additional calcium supplementation of 1000 mg daily increased the rate of bone mineral density in prepubescent children, even when their mean dietary intake approximated the RDA (source). A bone mineralization study determining bone mineralization during growth showed that size-adjusted bone mineral content in school-aged children was positively associated with average calcium intake; however, size-adjusted accretion in bone mineral content was only positively associated with changes in the dietary calcium intake in boys (source). Although there is no convincing evidence that young girls need more than 900 mg of calcium daily to achieve peak bone mass, it is only the dose, not a requirement for calcium, that is unclear (source). Studies have also shown that dietary calcium slows osteoporotic bone loss, and the preventive effect of calcium supplements is most evident in women more than 10 years after menopause (source). In Europe, low calcium intake and suboptimal vitamin D status are common in the elderly, and evidence indicates that routine daily supplementation is beneficial for people at risk for osteoporosis with 700 to 800 mg of calcium and 400 to 800 IU of vitamin D (source).
Calcium has a protective effect on bowel epithelium; it may precipitate toxic bile acids in the colonic lumen, thus reducing the rate of proliferation of colonic epithelium. Calcium has been found to protect against colon carcinogenesis in animal models, and a human intervention trial demonstrated that supplementation with 1200 mg of calcium carbonate per day led to a significant reduction in the risk of recurrent colonic adenomas (source). Those with a high dietary intake of calcium have been found to have a lower risk for colon cancer in both men and women (source). In people with adenoma, calcium supplementation (1.5 g daily) over 12 months was found to significantly suppress rectal epithelial proliferation (source). Though, a randomized intervention trial indicated that calcium supplementation (2.0 g) was associated with a modest but non-significant reduction in the risk of adenoma recurrence (source), it should be noted that long-term dietary habits influence the outcome of these studies. Supplementation with fibre, such as 3.5 grams of ispaghula husk, may have adverse effects on colorectal adenoma recurrence, especially in patients with high dietary calcium intakes. Calcium is also postulated to affect the risk of cancer at other sites. High calcium concentrations in drinking water correlate with a significantly reduced risk of breast cancer (source), and analysis of data from the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study suggests a possible association between the interaction of calcium and phosphorus with the risk of prostate cancer (source).
Interestingly, while hypercalcemia is associated with hypertension, calcium supplementation to RDA levels may lower blood pressure. Those with a calcium intake of less than 700 mg daily tend to have higher blood pressure. Results of epidemiologic studies consistently indicate an inverse relationship between dietary calcium intake and blood pressure, but the results of clinical trials with calcium supplements have been less consistent. Nonetheless, recommendations from the Sixth Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure include increasing calcium, potassium, and magnesium intake; reducing refined sodium intake; controlling obesity; and avoiding heavy alcohol intake, along with aggressive blood pressure control (source).
Calcium is important for blood pressure control during pregnancy. A randomized trial of pregnant women at high risk of developing preeclampsia, showed that 2 g of calcium daily was helpful in preventing preeclampsia (source). This is consistent with a review of 9 studies that were all of good quality, which suggested a modest reduction in high blood pressure and the risk of preeclampsia with calcium supplementation (source). The reviewers concluded that calcium supplementation appeared to be beneficial for women at high risk of gestational hypertension and in communities with low dietary calcium intake. In another review, with 13 high-quality studies, greater than 1 gram of calcium supplementation per day was associated with a significant reduction in the risk of pre-eclampsia, particularly for women with low calcium diets (source). The authors concluded that the benefits outweigh the increased risk of HELLP (haemolysis, elevated liver enzymes and low platelets) syndrome, which was small in absolute numbers. Where high-dose supplementation is not an option, lower-dose supplements (500 to 600 mg/day) might be considered in preference to no supplementation in settings of low dietary calcium (source).
Human studies have also shown that calcium depletion appears to be an important consequence of potassium depletion in subjects with hypertension and a higher salt sensitivity index (source). Therefore treatment of salt-sensitive patients with hypertension may best be undertaken with refined sodium restriction, in addition to potassium and calcium supplementation. Although the mechanism by which calcium reduces blood pressure requires elucidation. Animal studies have shown that calcium supplementation reduces blood pressure during chronic nitric oxide synthase inhibition and abrogates the associated impairments in endothelium-dependent and endothelium-independent arterial relaxation (source). It has also been postulated that increased calcium intake may improve calmodulin activity, which has the potential to interfere with various cellular processes linked to vascular tone (source).
Increased calcium intake is also associated with the following:
Reduced risk of nephrolithiasis. Calcium possibly prevents kidney stones by reducing urinary oxalate content (source).
Reduced symptoms of premenstrual tension. Premenstrual syndrome may represent the clinical manifestation of a calcium deficiency state that is unmasked after the increase in ovarian steroid hormone concentrations during the menstrual cycle (source).
A prospective, randomized, double-blind, placebo-controlled, parallel-group, multicenter clinical trial demonstrated that supplementation with 1200 mg of elemental calcium daily resulted in a major reduction in overall luteal phase symptoms of premenstrual tension (source, source, source).
An improved serum lipid profile. Calcium may increase excretion of saturated fats and reduce serum low-density lipoprotein cholesterol and apolipoprotein B levels (source).
Hair Tissue Mineral Analysis Notes (to be updated):
Hair calcium levels are very interesting to me. I have found that many people have symptoms of calcium imbalances such as insomnia that have near ideal calcium or at least below 80mg%.
High hair calcium concentration is associated with low calcium intake and low bone mineral density (BMD) (source). Rickets/osteomalacia together with suspiciously high toxic levels of Cd (or perhaps poor elimination) and various other elements (such as iron, manganese, magnesium, strontium and barium) have been observed (source).
While very low hair calcium concentration has been observed in women over 62 years of age related to senile osteoporosis and hypoparathyroidism (source). It appears that both high and low calcium levels on a hair test are indicative of osteoporosis and impaired bone metabolism. When assessing bone health with a hair tissue mineral analysis, calcium should be evaluated in relationship to phosphorus, magnesium, sodium and potassium and other minerals such as zinc, copper, and manganese that are necessary for bone health (source).
High hair calcium typically indicates a slow metabolism and that calcium is leaving the bones and accumulating in the soft tissues of the body. It is a good indicator for copper toxicity. Higher calcium level on a retest often means the body is eliminating excess calcium and should not be interpreted as a slowing of the oxidation rate as long as the Na/K is the same or has improved.
Low hair calcium typically indicates a fast oxidation rate and that calcium is being lost in the urine which is often associated with copper deficiency.
Toxicity/Drug interactions:
One of the most commonly associated symptoms of calcium supplementation is constipation. Calcium supplements are poorly absorbed when taken without vitamin D3. Vitamin D3 (cholecalciferol) increases calcium absorption, although vitamin D2 (ergocalciferol) does not. Excessive cholecalciferol and calcium supplementation may cause hypercalcemia.
Hypercalcemia presents clinically with the following signs symptoms:
weakness, dehydration, metastatic calcification, impaired concentration, increased sleep, altered state of consciousness, polydipsia, anorexia, nausea, vomiting, constipation, pancreatitis, peptic ulceration, polyuria, nephrolithiasis, nephrocalcinosis, and hypertension.
In the absence of vitamin D supplementation, hypercalcemia is unlikely. Oral calcium supplements may interfere with other medications if they are taken within 2 hours of each other. Supplementation with 1800 mg of calcium gluconate decreases net zinc absorption from a balanced diet that supplies 216 mg of calcium and 13.1 mg of zinc. When calcium is consumed in the form of calcium carbonate (an antacid), it may impair the absorption of other minerals such as iron and zinc, acetaminophen, phenytoin, and thyroid hormones. Although calcium carbonate is less expensive, the bioavailability of marketed calcium carbonate is equivalent to that of calcium citrate (source).
Clinical Caution:
Mild calcium deficiency may present as anxiety, irritability, and insomnia. More severe deficiency presents as muscle cramps and palpitations in the short term and as stunted growth, osteoporosis, and rickets in the long term. Persons at risk are those with lactose intolerance and those avoiding dietary dairy products.
There has been some concern regarding lead in calcium supplementation (source, source). Calcium supplements manufactured according to US Pharmacopeia (USP) requirements have a standardized dose and are without significant impurities (source, source, source).
Calcium interferes with the absorption of some pharmaceuticals such as bisphosphonates like alendronate (Fosamax), risedronate (Actonel), and once-monthly ibandronate (Boniva). Thus, those taking one of these agents should be told to wait at least 30 minutes before taking calcium supplements (and 60 minutes with ibandronate) (source, source, source).
Practice Tips:
Children, adolescents, and pregnant, lactating, perimenopausal, or postmenopausal women may need more calcium than they normally obtain from eating calcium-rich foods.
Always consider the absorption rate of the different forms of calcium when recommending dose. My preference at the moment is MCHC.
Dolomite should not be used as sources of calcium, because these products may contain lead.
Always look for USP certified calcium supplements.
The elemental calcium content of a supplement should always be ascertained because the proportion of calcium and the absorption varies among compounds.
When long-term calcium supplementation is prescribed, the patient’s magnesium status should be checked.
Water softeners remove calcium from water.
Calcium can temporarily control cardiac arrhythmia associated with hypokalemia.
Nocturnal cramps may be relieved by administration of 4.8 g of calcium gluconate at bedtime.
Persons whose urine turns pinkish after eating 250 g of beetroot may have hypochlorhydria and thus impaired calcium absorption.
Hypercalcemia should be suspected in people receiving calcium and vitamin D supplementation who complain of a dry mouth, persistent headache, irritability, anorexia, and depression.
Drinking 3 cups of milk is helpful, but does not reach the RDI of 1,200mg when considering the 30% absorption rate.
Supplementation can slow the oxidation rate. But may be needed in conjunction with nutrients that raise the sodium/potassium (Na/K) ratio.
Supplementation helps detoxify lead (source) and cadmium.
Supplementation helps to balance key mineral ratios and reduce sympathetic nervous system activity
Additional Reading:
http://www.rsc.org/periodic-table/element/20/calcium
http://raypeat.com/articles/articles/calcium.shtmlhttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC3780531/